Research Excellence and Intensity
CMAC’s leading advanced pharmaceutical manufacturing research programme will deliver manufacturing technologies that enable industry to deliver better products, quickly, economically and sustainably. This meets the industry demand for reduced time and costs for pharmaceutical development.
Research Programmes
-

Digital CMC CERSI
The Digital CMC CERSI is a centre of excellence to accelerate the adoption of digital technologies in regulatory processes to ensure faster, more efficient delivery of medicines to patients with reduced environmental impact.
-

MediForge
MediForge will research and develop new innovations to overcome the limitations of traditional Chemistry, Manufacturing, and Control (CMC) development processes which can be costly, time consuming and inflexible.
Find out more
-

Digital Medicines Manufacturing Research Centre (DM²)
DM² aims to transform medicines development and manufacturing productivity, and drive patient-centric supply.
-

Cyber-Physical Systems for Medicines Manufacturing (CEDAR)
CEDAR aims to develop the way that cyber-physical systems can help make medicines manufacturing more sustainable, resilient and human-centric.
-

Future Manufacturing Research Hub
The Hubs goal is to address the Grand Challenge “Digitally Enabled MicroFactory Based Medicines Manufacture and Supply”.
-

CMAC Data Lab
The CMAC Data Lab will be a world-first Industry 5.0 research facility that will support a critical mass of new research that will revolutionise the way we design, develop, manufacture, and regulate medicines through the digitalisation of CMC processes.
-

Digital Design and Manufacture of Amorphous Pharmaceuticals (DDMAP)
DDMAP aims to address key research questions that underpin the selection, production, and application of amorphous molecular solids in medicines development and manufacture.
-

GI Bio
GI Bio addresses a major challenge in understanding the gut's complex dynamic environment by offering a way to replicate it without the limitations of animal or human studies.
-

MMIC Grand Challenge 1
The Medicines Manufacturing Innovation Centre is a collaboration between CPI, University of Strathclyde, UK Research & Innovation, Scottish Enterprise and founding industry partners, AstraZeneca and GSK.
-

Hub Feasibility Studies
CMAC has supported 4 feasibility projects at different universities across the UK. The feasibility projects have been selected to align with the Hub goals and address specific areas of interest.
-

CMAC Industry Core Projects
Pre-Competitive Core Projects are funded jointly by the Tier 1 companies and are designed to develop translatable outputs to deliver impact in research areas aligned with CMAC.
-

ARTICULAR
The ambitious ARTICULAR Programme will develop novel AI approaches for pharmaceutical manufacturing. Machine learning from development and manufacturing data and models is used to address common pharmaceutical product development problems.
Collaborative Projects
We work closely with Academic and Industrial partners in a range of collaboratively funded projects
Digital
Advanced Characterisation
Innovation & Engagement
Scale-Up
UK Pharmaceutical Manufacturing Landscape
CMAC is a key part of the UK Medicines Manufacturing landscape.
CMAC’s focus will be to deliver novel manufacturing technology that will enable industry to deliver better products, quickly, economically and sustainably. This will meet the demand for reduced development time and costs and to exploit emerging opportunities driven by the urgent needs of patients and consumers for more personalised product performance.
As part of critical regulatory agenda CMAC and MIT organise a biennial conference with the FDA.
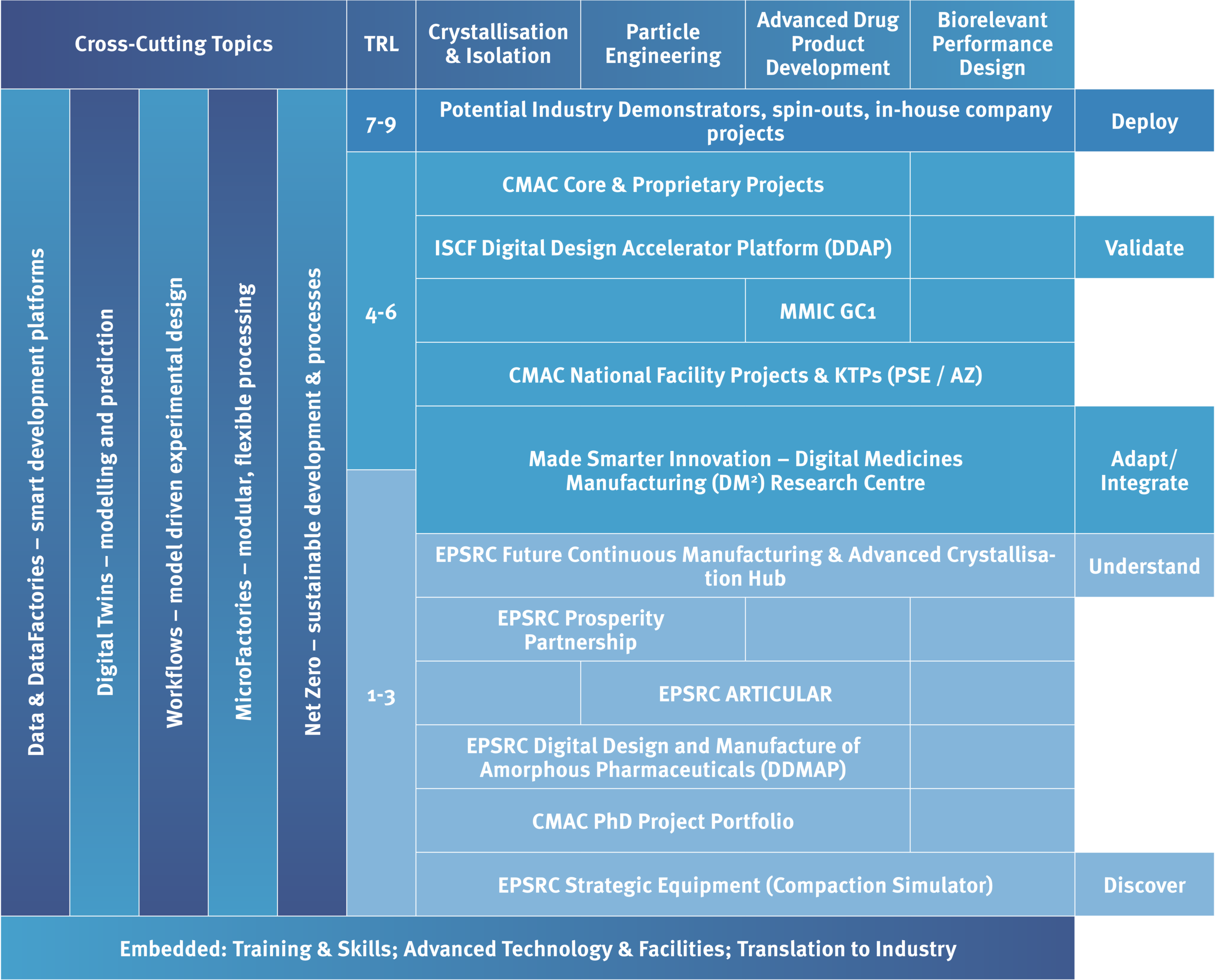
Research Portfolio
CMAC’s leading advanced pharmaceutical manufacturing research programme currently covers TRLs 1-6. The aligned portfolio of projects - supported by EPSRC, InnovateUK, and industry - gives a group of partners with complementary expertise to deliver research with impact.
The CMAC research portfolio comprises of around 75 projects that cover the CMAC research themes, including:
Quality by Digital Design,
Digital Twins,
Workflow and model development,
Advanced measurement techniques,
Crystallisation Classification System (CCS), Manufacturability Classification System (MCS), Biorelevance Performance Classification System (BPCS),
Primary and secondary MicroFactory processing, and supply chain mapping
2021 CMAC Portfolio by Scope